New Delhi: Advancing research into mental disorders and brain development, Stanford Medicine investigators have successfully connected living human nerve cells, or neurons, and supporting brain cells with the brain tissue of rats to form hybridized working circuits.
The research, described in a study published online Oct. 12 in Nature, demonstrates a method for performing experiments that would otherwise be invasive, difficult or impossible. By growing and manipulating human brain tissue in the living laboratory of a rat’s brain, researchers can observe effects on the animal’s behavior, said Sergiu Pasca, MD, professor of psychiatry and behavioral sciences at the Stanford School of Medicine.
Advancing research into mental disorders & brain development, Stanford Medicine investigators have successfully connected living human nerve cells, or neurons, and supporting brain cells with the brain tissue of rats to form hybridized working circuits. https://t.co/gVcT7yuakW
— Stanford Medicine (@StanfordMed) October 12, 2022
---Advertisement---
“We can now study healthy brain development as well as brain disorders understood to take root in development in unprecedented detail, without needing to excise tissue from a human brain,” said Pasca, the Bonnie Uytengsu and Family Director of Stanford Brain Organogenesis. “We can also use this new platform to test new drugs and gene therapies for neuropsychiatric disorders.”
Also Read :- Elon Musk to not withdraw Starlink internet service funding in Ukraine
Pasca is the study’s senior author. Lead authorship is shared by former postdoctoral scholar Omer Revah, PhD, DMV; basic life research scientist Felicity Gore, PhD; and psychiatry resident and postdoctoral scholar Kevin Kelley, MD, PhD.
Ingredients for success
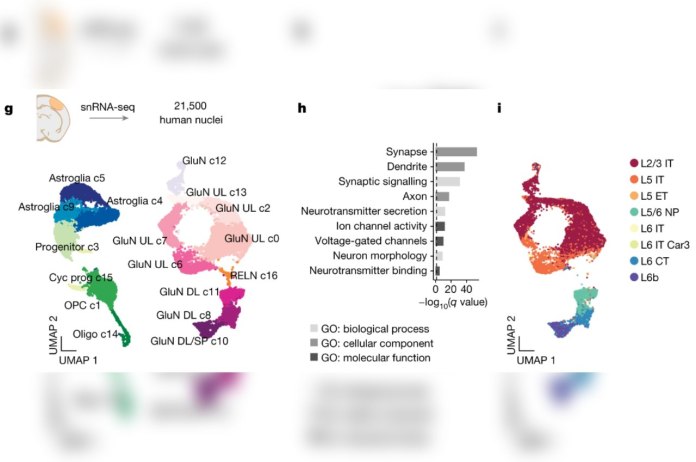
The investigators began by using a method Pasca first reported in Nature Methods in 2015. In that study, human skin cells were first transformed into stem cells, which can differentiate into most of the body’s 200 or so cell types. Under carefully guided conditions in laboratory dishes, these cells differentiated into several brain-cell types and multiplied to form organoids resembling the cerebral cortex — the human brain’s outermost layer as well as its most recently evolved part.
Variations in this protocol have enabled Pasca and his colleagues to generate organoids representing a dozen distinct brain regions.
“We’ve been making ever more complicated circuits in a dish using organoids and sophisticated combinations of them, called assembloids. But neurons within these lab dishes are still lagging behind in their development compared with what you’d see in a naturally developing human brain,” Pasca said. Numerous challenges — such as a lack of nutrients and growth factors, blood-vessel-forming endothelial cells or sensory input — hinder development in a lab dish, he said.
The transplantation recipients in the study were rat pups a mere two to three days old — the equivalent of infancy in a human. Performing an identical procedure using adult rats would have been extremely unlikely to have paid off, Pasca said, because brain connections are largely formed early in development. The brain becomes substantially less permissive to forming more connections after these early, critical periods have passed, he said.
After two months or so in culture, organoids closely resembling the human cerebral cortex were transferred into the brains of young rats — close to 100 over the course of the study. The organoids were placed in the same location in each rat’s brain so their development could be more easily monitored.
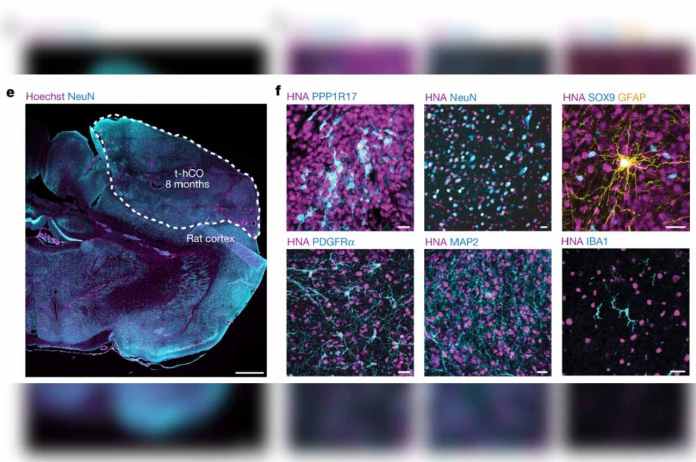
Cells from the rats soon migrated into the human tissue: Rat endothelial cells penetrated the human brain implants and assembled into blood vessels, which supply nutrients and signaling substances to the human cells and cart away waste metabolites. Resident immune cells in the rat brain also populated the human graft.
“They moved right in,” Pasca said.
The implanted human organoids survived, thrived and grew. Measuring perhaps one-fifth of an inch when transplanted, they expanded to the point where, six months later, they occupied a full one-third of the hemisphere of the rat brain into which they’d been implanted.
Individual neurons from the human organoids in the rat brains were now at least six times as big as those in the organoids — generated the same way, at the same time — that remained in a dish instead of being transplanted. They also exhibited much more sophisticated branching patterns.
Neurons from the organoids set up shop in the rats’ brains, forming working connections with indigenous circuits. One of the rat brain structures with which the human neurons forged direct connections was the thalamus, a region deep in the brain that relays multiple sensory inputs to the cortex.
“This connection may have provided the signaling necessary for optimal maturation and integration of the human neurons,” Pasca said.
Also Read :- ‘Rupee is not depreciating, dollar is rising’: Nirmala Sitharaman in DC
The ultimate controlled experiment
To gauge the transplanted organoids’ capacity to flag molecular underpinnings of human neuropsychiatric disorders, Pasca and his colleagues turned to Timothy syndrome, a rare genetic condition strongly associated with autism and epilepsy. The scientists transplanted an organoid generated from a Timothy syndrome patient’s skin cells into one side of the rat’s brain. Into the corresponding spot on the other side, they transplanted an organoid generated from a healthy individual, who served as a control.
Five to six months later, the researchers observed marked differences between the two sides’ electrical activity. The Timothy syndrome neurons were also much smaller and were deficient in sprouting branching, brush-like extensions called dendrites, which act as antennae for input from nearby neurons.
“We’ve learned a lot about Timothy syndrome by studying organoids kept in a dish,” Pasca said. “But only with transplantation were we able to see these neuronal-activity-related differences.”
Similar comparisons — transplanting organoids from people with, say, schizophrenia or autism, versus those without those disorders, into opposite sides of the same rat’s brain — may reveal size, complexity, functional and connectivity differences, Pasca said.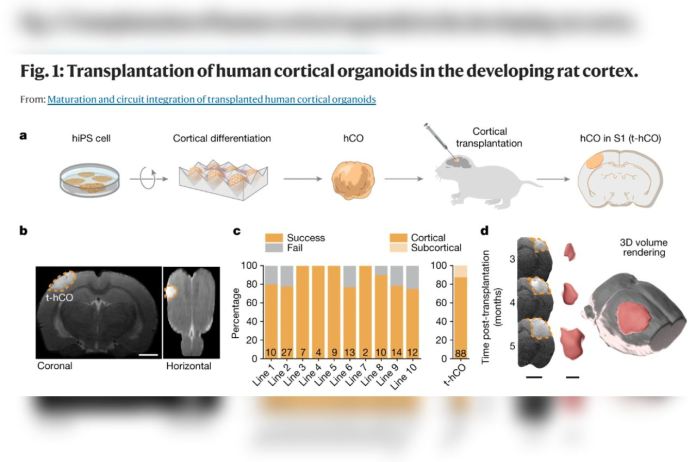
Pavlov’s rats
The Stanford Medicine researchers had taken care to position the organoids in the region of the rats’ brains that processes sensory information from the animals’ whiskers. The long hairs protruding from their snouts detect nearby objects and surfaces, helping them avoid collisions when they’re moving quickly or in unfamiliar places.
The scientists showed, definitively, that human neurons could be activated by input from rats’ senses. When they perturbed the now-adult rats’ whiskers with puffs of air, the human neurons in the rats’ brains became electrically active, responding in synchrony to individual air puffs.
Cognitive tests roughly 200 days post-transplantation showed that the rats were no more fearful than control rats, retained similar memory capabilities and did not experience seizures.
“Overall, we saw neither improvements nor deficits,” Pasca said.
In another experiment, the scientists implanted human organoids that had been modified so their constituent neurons could be activated by specific frequencies of blue laser light. About three months later, the investigators inserted ultrathin fiber-optic cables, which can transport laser light from a remote origination point to the human organoids in the rats’ brains. These rats were then placed in a glass box with a tiny water spout. The researchers employed a form of Pavlovian conditioning by delivering random bursts of blue light to activate human-organoid tissue inside the rats’ brains — but making water available to the rats only after blue-light pulses. By the end of a 15-day training period, simply pulsing blue light into the organoid was enough to send the rats scurrying to the spout.
The rats had learned to associate blue-light stimulation of their engrafted human neurons with the reward of water, proving that the implanted human tissue had integrated into and could engage the rats’ reward-seeking circuits — a complex of neuronal pathways that, in all mammals, guides behavior toward activities associated with previous pleasant outcomes.
“This is the most advanced human brain circuitry ever built from human skin cells and a demonstration that implanted human neurons can influence an animal’s behavior,” Pasca said. “Our platform provides, for the first time, behavioral readouts for human cells and could, we hope, accelerate our understanding of complex psychiatric conditions.”
Also Read :- ‘Saddened at non-extension of truce in Yemen’: India at UNSC
The study was funded by the National Institutes of Health (grants R01MH115012, K99DA050662 and S10RR026917-01), Stanford Wu Tsai Neuroscience Institute, the Stanford Brain Organogenesis Program, Bio-X, the Kwan Funds, the Senkut Funds, the New York Stem Cell Foundation, the Chan-Zuckerberg Initiative, the Coates Foundation, the Ludwig Family Foundation and the Alfred E. Mann Foundation.
Annotations for the images above














