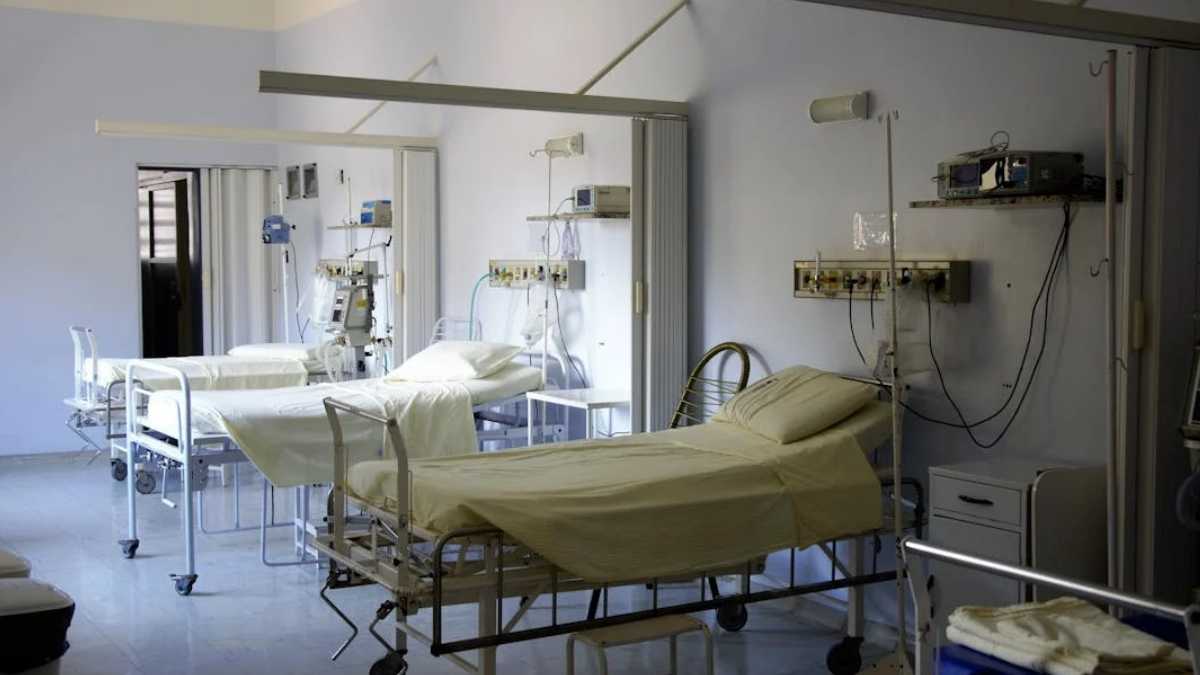
A major healthcare scandal has come to light in West Bengal, as the Directorate of Health Services (DHS) reveals the supply of substandard Oximetry Central Venous Catheters (CVCs) to government hospitals by a multinational medical device company – Vygon India Pvt. Ltd. This “Catheter Scam” poses concerns over patient safety and regulatory oversight within the healthcare sector.
The story began when hospital staff at facilities like Kolkata Medical College and SSKM started noticing unusual discrepancies in the quality of catheters they were receiving through patient complaints and their observations. Central Venous Catheters play a crucial role in administering medication and monitoring patients, particularly in high-stakes situations. By the time the issue was flagged, a significant number of these substandard catheters had already been distributed, risking the health of numerous patients in critical care units.
In response to the allegations, Vygon India has denied any wrongdoing, attributing the malpractice to its distributor, Prakash Surgicals. However, concerns about the lack of oversight by the principal company are mounting and the doubts of such a large scam happening without the connivance of its top management are growing.
In a move that some see as a step toward justice, the DHS has banned Vygon India from participating in Central Medical Stores (CMS) tenders for two years. Yet as welcome as this action is, a few questions arise, is this enough? Is it adequate compensation for the established charges which have as per the government order “impacted the repute of the government supply in the hospitals” and “tarnished the repute of the health system”.
Multinational companies are insured. Has the West Bengal government considered suing for damages and if not why a lenient view towards a company which is charged for tarnishing the government’s reputation and damaging its patients? Shouldn’t there be an assessment of those patients who suffered due to the catheters that malfunctioned and they also be compensated for the damages sustained? Shouldn’t there be stricter action to save innocent patients from such life-threatening frauds again? Since Vygon has its operations in all states and UTs of India have these states been alerted to this fraud and the risk? Has CDSCO been informed?
There is growing fear that this scam could extend well beyond West Bengal, revealing a broader network driven by profit. Hospitals across India might have unknowingly utilized these substandard catheters, potentially leading to severe complications or fatalities among patients relying on critical care.
It calls for a comprehensive investigation into the distribution and corporate governance practices by central agencies like the Central Drugs Standard Control Organization (CDSCO) and the Department of Pharmaceuticals to delve deeper into Vygon India and their operations in other states. At its core, the “Catheter Scam” is more than just a story of corporate negligence; it’s a wake-up call for vigilance authorities to corner the wrong doers, find the extent of wrong doings and curtailing their fall out on unsuspecting patients of our country.